Green Methanol: A Sustainable Future
by J Srikanth, Dr S Sakthivel · Published · Updated

Introduction
Methanol, also known as methyl alcohol or wood alcohol, is a vital chemical compound with the formula CH₃OH. It is a colourless, volatile liquid that is widely utilised across various industries. The simplicity of its chemical structure, a methyl group bonded to a hydroxyl group, makes methanol versatile. It serves as a feedstock for the production of a range of chemicals, including acetic acid, formaldehyde, and methyl tert-butyl ether (MTBE). These are key components in the pharmaceutical, plastics, textile, and automotive industries. Methanol is also used as fuel for fuel cells, a hydrogen carrier, in biodiesel production, and as a solvent in paints and coatings. However, while its broad applicability is undeniable, methanol’s toxicity when ingested or absorbed through the skin necessitates stringent safety measures.
The growing interest in reducing carbon emissions and fostering sustainability has spurred innovation in methanol production, leading to the emergence of green methanol, which is considered crucial for the transition to carbon-neutral energy sources.
Pathways to Methanol Production
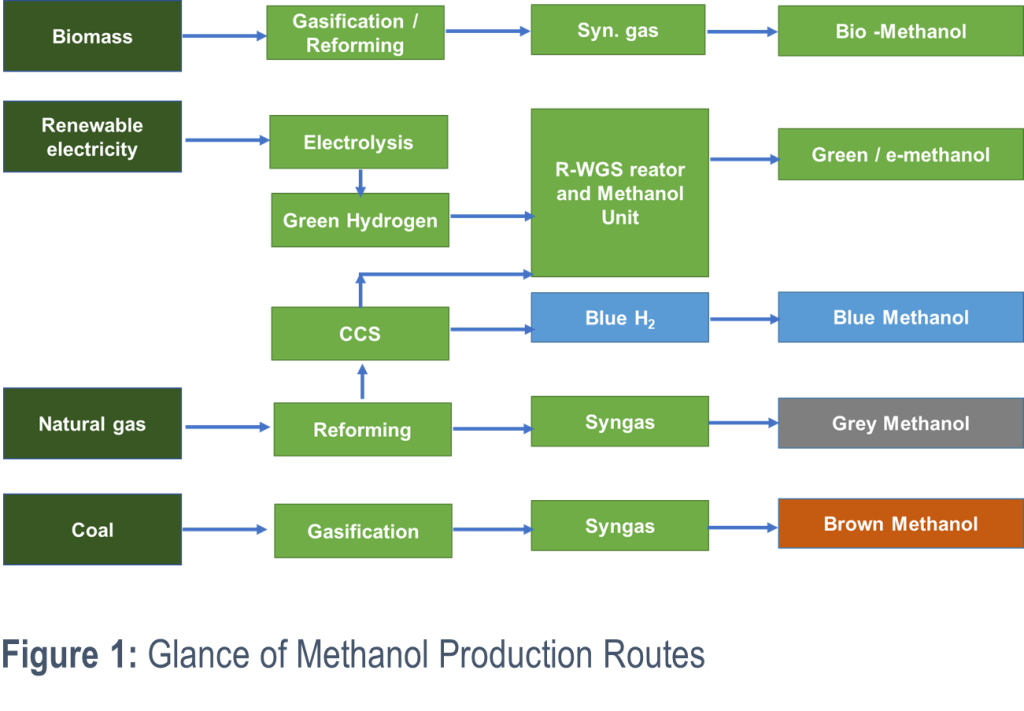
Methanol production can follow different routes, each with distinct environmental impacts. These routes are classified by colour codes representing their carbon footprints:
- Grey Methanol: Produced via steam methane reforming of natural gas, grey methanol is carbon-intensive due to the release of significant amounts of CO₂.
- Brown Methanol: Created through coal gasification, this traditional method of methanol production also generates high levels of CO₂, contributing heavily to environmental concerns.
- Blue Methanol: Similar to grey methanol but incorporates carbon capture technology to sequester emissions, making it a cleaner alternative.
- Bio-Methanol: Derived from biomass such as agricultural residues and waste through gasification, bio-methanol provides a renewable and more sustainable production route.
- Green Methanol (e-Methanol): Produced by combining captured CO₂ with green hydrogen generated from renewable energy sources. Green methanol offers a pathway to carbon neutrality, aligning with sustainability goals and efforts to combat climate change.
Traditional methanol production methods, relying on fossil fuels such as natural gas and coal, contribute substantially to greenhouse gas emissions. On average, producing one tonne of methanol via conventional methods emits 1.6 tonnes of CO₂. With over 110 million metric tonnes of methanol produced annually worldwide, predominantly using fossil fuels, the industry is a significant source of emissions. Only a small fraction of global methanol production comes from renewable sources, highlighting the need for innovative approaches like green methanol.
Green Methanol: A Step Towards Sustainability
Green methanol, or e-methanol, is at the forefront of sustainable methanol production. This process involves synthesising methanol by reacting captured CO₂ with hydrogen produced from renewable sources, such as solar or wind energy. Green methanol offers several key environmental benefits:
- Reduction in CO₂ Emissions: By using captured CO₂, green methanol significantly reduces the carbon footprint compared to conventional production methods.
- Carbon Neutrality: The process of capturing CO₂ and reusing it in methanol production closes the carbon loop, making green methanol a potential carbon-neutral energy carrier.
- Enhanced Sustainability: With renewable hydrogen as a key input, green methanol aligns with the broader push towards a sustainable, low-carbon future.
The Green Methanol Production Process
The production of green methanol is a multi-step process involving carbon capture, hydrogen generation, and methanol synthesis:
- Carbon Capture: CO₂ is captured from industrial emissions or directly from the atmosphere using technologies like absorption or membrane separation.
- Hydrogen Generation: Renewable energy is used to electrolyse water, generating green hydrogen.
- Methanol Synthesis: CO₂ and hydrogen are fed into a reactor, where a catalytic reaction produces methanol (CH₃OH). The catalysts commonly used in this process are based on copper, zinc, and aluminium oxides, which facilitate the reaction at temperatures of 210–270°C and pressures of 5–10 MPa.
The chemical reaction for methanol production is as follows:
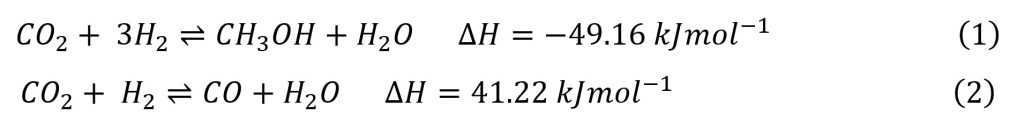
For every tonne of methanol produced, approximately 1.37 tonnes of CO₂ and 0.19 tonnes of hydrogen are required, with water as a byproduct. The crude methanol is purified through distillation to ensure high quality.
Challenges in Green Methanol Production
While green methanol holds immense promise, several challenges remain:
- Cost: Producing green methanol is currently more expensive than traditional methods, largely due to the high cost of hydrogen generation through electrolysis.
- Infrastructure: Existing infrastructure for methanol storage and distribution may need to be adapted to accommodate large-scale green methanol production.
- Efficiency: Enhancing the efficiency of carbon capture, hydrogen production, and methanol synthesis is essential to improve the energy yield and reduce costs.
- Policy Support: Governments must implement supportive policies, such as subsidies or carbon pricing, to encourage the adoption of green methanol production technologies.
Despite these challenges, advancements in technology and increasing environmental awareness are driving investment and innovation in the green methanol sector.
The Role of Tata Consulting Engineers (TCE)
Tata Consulting Engineers (TCE) is at the forefront of engineering solutions for carbon capture and utilisation. TCE’s expertise extends to the conversion of captured CO₂ into valuable products, including methanol, which is widely used across industries, and sustainable aviation fuel. TCE’s innovations in carbon capture technology ensure that environmental impact is mitigated while providing economically viable resources for various industrial applications.
Conclusion
Green methanol represents a transformative step towards a sustainable and low-carbon future. By leveraging captured CO₂ and renewable hydrogen, green methanol production offers a path to carbon neutrality, addressing both energy sustainability and emissions reduction. As technology continues to evolve and policy frameworks support the transition, green methanol is poised to play a significant role in the global energy landscape, helping pave the way for a cleaner, more sustainable future.
References
- IRENA. 2021. Innovation Outlook: Renewable Methanol. IRENA, Abu Dhabi.
- Wang, D., Meng, W., Zhou, H., et al. 2022. Novel Coal-to-Methanol Process with Near-Zero Carbon Emission. J. Clean. Prod., 339.
- Anetjärvi, E., Vakkilainen, E., Melin, K. 2023. Benefits of Hybrid Production of e-Methanol in Connection with Biomass Gasification. Energy, 276.
- Sollai, S., Porcu, A., Tola, V., et al. 2023. Renewable Methanol Production from Green Hydrogen and Captured CO₂: A Techno-Economic Assessment. Journal of CO₂ Utilisation, 68.
- Stangeland, K., Li, H., Yu, Z. 2020. CO₂ Hydrogenation to Methanol: Structure–Activity Relationships of Different Catalyst Systems. Energ. Ecol. Environ., 5.





