Hydrogen Production Technology

Hydrogen (H2) is stated as sustainable alternative energy to fossil fuels, especially in e-transport, the source of industrial energy and feedstock, and medium for energy storage. Generally, hydrogen is produced (about 96%) from fossil fuels like natural gas, crude oil and coal. Nowadays, energy producers mainly focus on carbon-free energy production systems to partially replace fossil fuel-based systems. Hydrogen is thus a clean-burning and emission-free fuel, which can be produced locally from fossil fuel or renewable energy sources [1].
Hydrogen has the highest energy density (120 MJ/kg) than gasoline (45 MJ/kg). Hydrogen ensures
- Energy security (i.e., produced from a variety of domestic sources, which reduces the country’s imports of oil/gas),
- Sustainability (renewable energy contributes to a great extent to H2 production),
- Climate change (i.e. contributes to clean energy transitions),
- Urban air quality and
- Economic vitality.
Hydrogen is an alternative clean fuel being explored in new applications such as transport fuel, power generation, building heat and energy, iron and steel making via direct reduction of iron (DRI) process, and as an energy storage medium. Hydrogen can be combined with other chemical molecules to produce ‘hydrogen-based fuels’ such as synthetic methane, ammonia, synthetic liquid fuels and methanol. These can be easier to handle than pure hydrogen and can be used as feedstock in industry.
Hydrogen Production Routes:
Several technologies have been recognised to produce hydrogen from non-renewable and renewable sources, as shown in Figure 1.
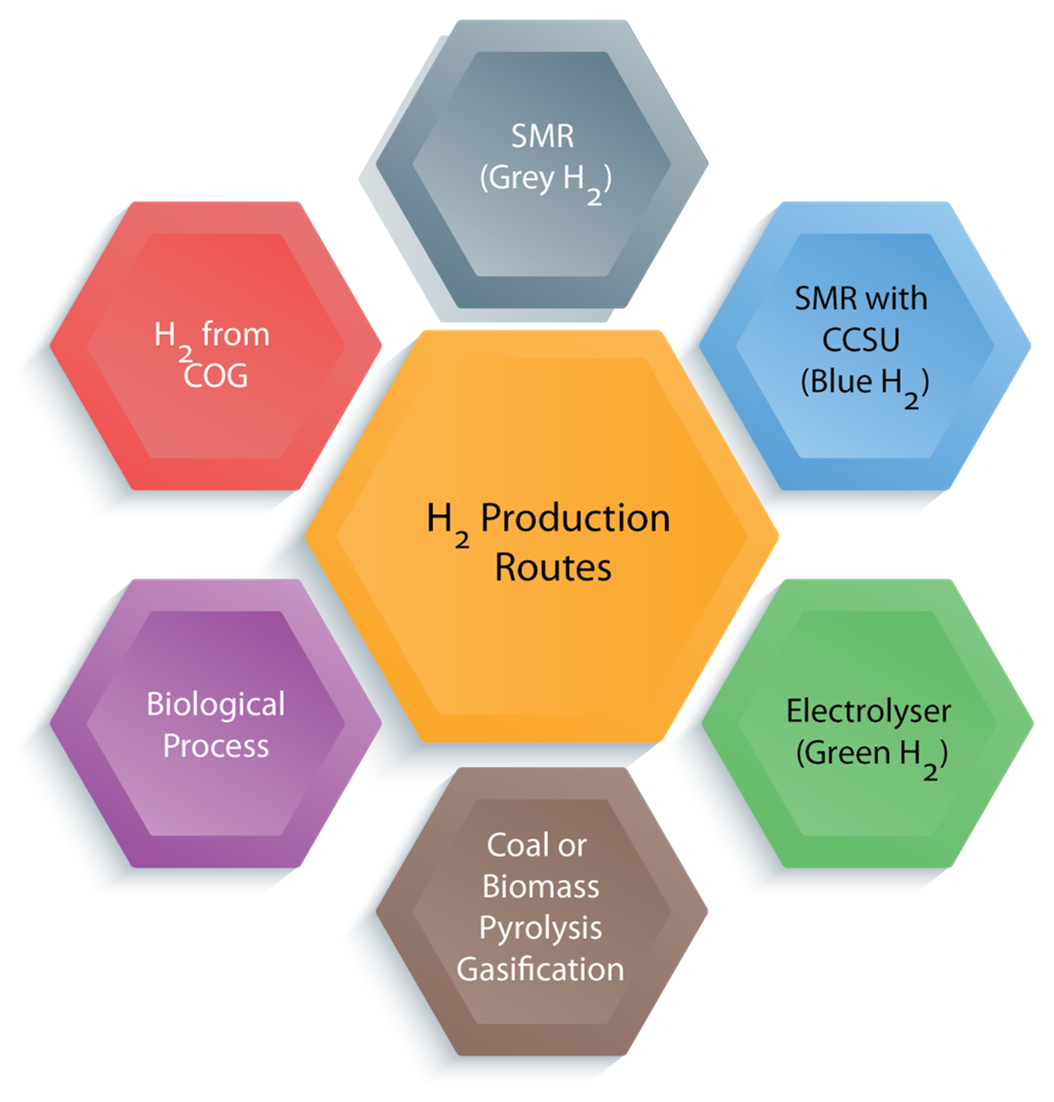
These are described below
- Steam Methane Reforming (SMR):The most well-known process for large-scale hydrogen production, with 74% – 85% conversion efficiency. More than 96% of hydrogen production is produced through steam methane reforming of natural gas or coal gasification [2]. SMR uses natural gas as feedstock, and this process releases high concentrations of greenhouse gases (~ 9 kg of CO2 per kg of H2 produced). However, it is the cheapest production route. The produced hydrogen is called grey hydrogen. It has few concerns, such as high CO2 emissions and the need for high capital and maintenance costs. Nowadays, Hydrogen producers are integrating carbon capture and storage (CCS) technology with the SMR process, which results in free-CO2 emission to the atmosphere. The produced hydrogen is called blue hydrogen.
- Electrolyser Technology: This has been the most acknowledged commercial water electrolysis technology for hydrogen production and has received significant attention globally. Water electrolysis employs an electric current to split water into hydrogen and oxygen molecules in an electrolyser. The electrolyser is connected with renewable power sources (like solar or wind), the resulting pollutant-free hydrogen produced, called green hydrogen. This green hydrogen shows an encouraging trend towards providing sustainable solutions in the long term than grey hydrogen. There are four technologies available for electrolysis of water such as
- Alkaline Water Electrolysis (AWE),
- Proton Exchange Membrane Electrolysis (PEM),
- Solid Oxide Electrolysis (SOE) and
- Anion Exchange Membrane Electrolysis (AEM) as shown in Figure 2.
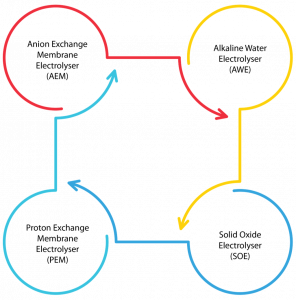
Globally, AWE and PEM Electrolyser operate both on commercial and pilot scales due to their technology maturity, durability and flexibility of low-temperature operation (<90 oC). AWE is a mature technology commercially available in the megawatt (MW) range, less expensive than PEM electrolysis. PEM is more beneficial than conventional alkaline electrolysis due to its smaller size, high purity (>99.99%), cleaner, more reliability, ecologically safe nature, and the possibility to produce at high pressure [1]. Currently, AWE and PEM are dominating the global market more than SOE. Typically, AWE and PEM consume specific energy consumption at 3.8 kWh/Nm3 and 4.53 kWh/Nm3, respectively.
- Pyrolysis / Gasification: Biomass derives from wood-based residues, dedicated energy crops, algae, agricultural waste and the organic portion of municipal solid waste and biosolids, such as sludge. These biomasses can be converted to hydrogen by a thermochemical process. Three thermochemical routes are available like gasification, pyrolysis and aqueous phase reforming. These processes typically consume 30 kg – 40 kg of biomass to produce 1 kg of hydrogen [2]. Biomass gasification is the thermal breakdown of biomass with oxygen at a temperature range of 800°C – 900°C at 1 bar – 33 bar. Typically, biomass gasification efficiency is approximately 52 %, but it depends on the type and quality of feedstock, the type of catalyst, the temperature level, etc. Biomass pyrolysis is the thermal breakdown of biomass in the absence of oxygen at 350°C – 550°C at 0.1 MPa – 0.5 MPa to produce biochar, bio-oil, and gas methane hydrogen, carbon monoxide, and carbon dioxide [2]. These processes have few concerns, such as higher CO2 emissions and tar and char formation leading to catalyst deactivation. H2 purity variation due to biomass complexity and composition variations and need for catalysts regeneration and high reactor cost [2].
- Biological Process: Hydrogen is produced from renewable sources like organic waste, algal biomass, and wastewater by photosynthetic or fermentative organisms. It is called as Bio-Hydrogen. It is known to be a more environment-friendly and less energy-intensive process than thermochemical and electrochemical processes. Bio-Hydrogen production technology can be classified through two significant pathways such as (a) Light‐dependent process (Bio-photolysis and Photo-fermentation) and (b) Light‐independent process (Dark fermentation and microbial electro-hydrogenises cells).
- Bio-photolysis: Green algae splits water molecules into hydrogen ions and oxygen ions via photosynthesis with hydrogenase enzyme. The hydrogenase enzyme helps to convert hydrogen ions to hydrogen. Several advantages of bio-photolysis are (a) it consumes CO2, (b) produces O2 as a by-product and (c) works under mild conditions. However, it has disadvantages such as low yields of H2, O2 sensitivity (<0.1%), sunlight is required, and large reactor size required. Typical bio-photolysis process efficiency is 10 to 11 %.
- Fermentation: This process provides an environmentally friendly bio-approach for the fermentative conversion of organic substrates into hydrogen. The organic feed materials are converted to H2, acetone and alcohols in minimal amounts and CO2 with or without oxygen by the microbial reaction. It’s a simple process, and it involves wastewater recycling and the release of used organic wastewater. The dark fermentation process is a breakdown of organic matter (like refined sugars, corn stover, and even wastewater) to produce hydrogen, volatile fatty acids, and CO2 under anaerobic conditions without the sunlight with the presence of heterotrophic bacteria and microalgae.
Typically, these processes operate at ambient temperature and atmospheric pressure, which is a less energy-intensive process. However, they have few concerns like low hydrogen yield and rate production, pre-treatment, and high by-product generation.
- Hydrogen from coke oven gas: Coke-Oven Gas (COG) is a by-product of coal carbonisation to coke in the coking process. COG is a combustible gas mixture that consists mainly of hydrogen and methane (CH4). Generally, 300−350 Nm3 of COG obtains per processing of one ton of dry coal, containing about 48 mol % of hydrogen. Thus, hydrogen can be recovered by using different technologies like (a) Chemical Absorption, (b) Cryogenic Separation, (c) Pressure Swing Absorption (PSA), (d) Membrane Separation and (e) Hydrate Separation. The brief information about these technologies in terms of significant energy consumption, operating conditions and separating efficiency are given in Figure 3 [3]
- Chemical absorption is suitable for the complete removal of specific impurities from crude hydrogen. This method consumes a high energy consumption process due to compression and desorption of gas. But it needs more than >6 MPa pressure.
- Cryogenic separation is a novel process for hydrogen separation from coke oven gas, which is under development stage. It requires high pressure (>3 MPa) and low temperature (-160 oC).
- The use of the PSA process has seen incredible growth during the last decades. PSA has some merits like low operating costs and its simplicity. It is capable of recovering high purity H2, CH4 and CO2 and the generation of N2 and O2.
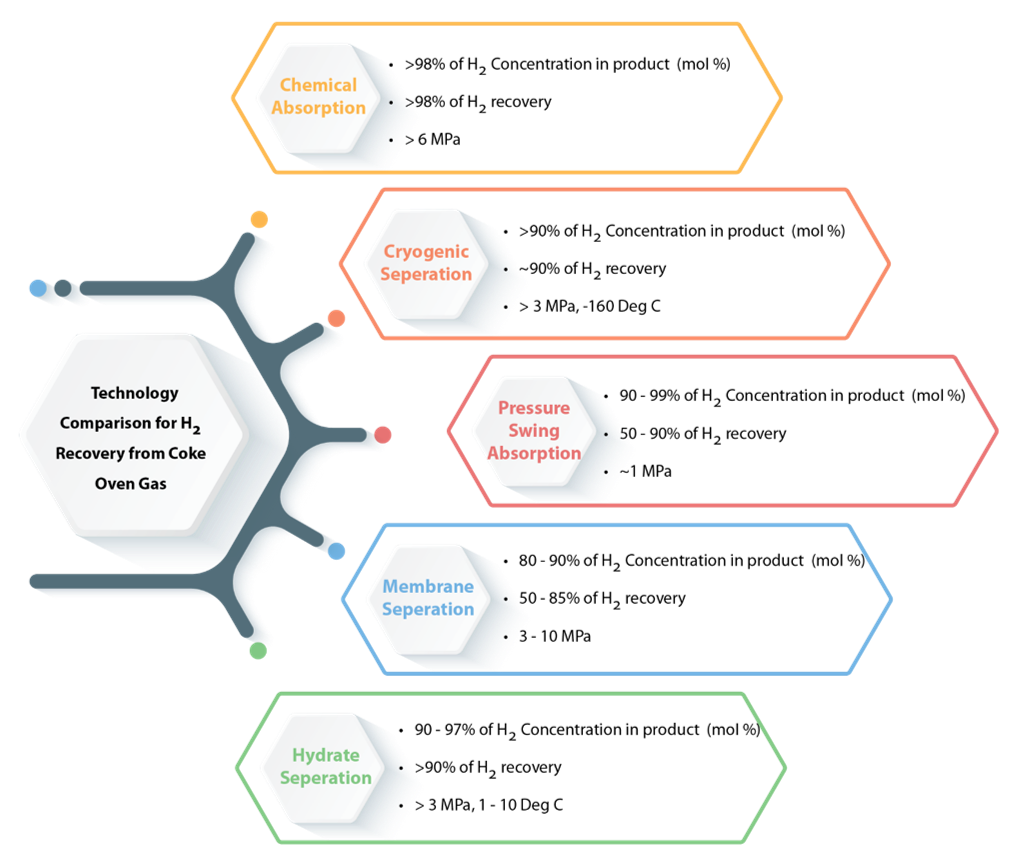
Figure 3 Technology comparison for H2 recovery from coke oven gas
- The membrane separation process is designed for the recovery of process gases from crude feed or off-gas streams. Often referred to as Hydrogen Recovery Unit (HRS), a membrane separation system is limited to hydrogen recovery. Also, the adjustment of synthesis gas ratios, recovery and separation of methane, helium, carbon dioxide, and oxygen and nitrogen from ambient air has to be mentioned as a few of many possible applications in the industry.
Technology Readiness Level (TRL) and Techno-economic Analysis:
Technology development will progress in a series of scale-up steps such as (a) Bench or Laboratory Scale, (b) Pilot-Scale, (c) Demonstration Scale, and (d) Commercial Scale. Figure 4 depicts the current hydrogen production technologies and their TRL scale [4]. Generally, TRL 3, TRL 6 and TRL 7 have bottlenecks for further development stages. TRL 3 requires additional research funding for progression to the next step. TRL 5 and TRL 7 needs significant financial investment and/or commercial interest for the subsequent stage of development.
Electrolysis of water is one of the promising technologies nowadays, which has reached TRL 9, but it is available at a small scale to mid-scale. The technology of thermolysis and photolysis are innovative approaches under the research stage, which has TRL 1 – 2. The biomass technology’s thermochemical (gasification/pyrolysis) has reached TRL 7, which needs further investment to commercialise. Biological and electrochemical processes have less TRL (4-5 and 2-4, respectively), which requires a lot of research to improve the yield.
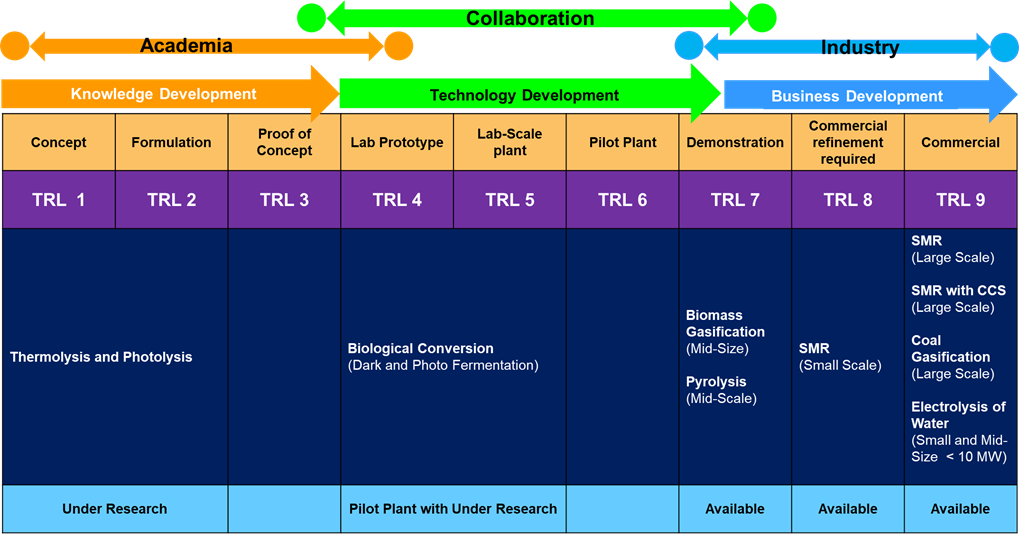
Figure 4 TRL level of various hydrogen production technologies
Generally, the cost of hydrogen is a function of the feedstock and operating cost to run the system. Typically, Capital Expensive (CAPEX) cost calculations include various components such as conversion units (like electrolyser, the balance of plant, stack replacement, project planning, deprecation period and interest rate). Operating Expensive (OPEX) costs includes direct operating costs of production (i.e., raw materials as water, specific electricity consumption, catalyst, KOH requirements, process steam, nitrogen and maintenance cost etc. are considered) [5]. Currently, SMR and coal gasification have the lowest hydrogen production cost (< 2 $/kg H2) due to existing large-scale infrastructures (TRL 9) and the low price of the feedstock. Biomass gasification and pyrolysis have reached TRL 7 at a mid-size plant. The hydrogen production cost is in the range of 1.21 -3.5 $/kg H2. However, it emits CO2, which is similar to fossil fuel feedstock. It has a few challenges, such as the type and quality of feedstock, reactor cost, and CCS systems combined with the processes. The water electrolysis process is an emerging technology to produce green hydrogen, but the cost of green hydrogen is high compared to the SMR process.
Closing Remarks
Hydrogen is emerging industrial energy and fuel as a clean and alternative source of fuel, which is fetching newer applications such as transport fuel, industrial feedstock, building heating energy, industrial energy and industrial power and stationary power generation. In this context, the hydrogen demand is also expected to increase, especially for ammonia and steel production and hydrogen-based vehicles. The expected hydrogen demand will be fulfilled by grey, blue and green hydrogen production routes. Engineering consulting firms need to play a significant role in this ambition to produce Grey, Blue and Green Hydrogen.
References
- S Sakthivel, Way forward to carbon-free electricity for e-mobility, Chemical Industry Digest, June-2019. http://chemindigest.com/way-forward-to-carbon-free-electricity-for-e-mobility/
- S Sakthivel, Hydrogen economy offers low-emissions fuel to combat air pollution, Gas Processing & LNG, Page 25-27, July/Aug 2020. http://www.gasprocessingnews.com/features/202008/hydrogen-economy-offers-low-emissions-fuel-to-combat-air-pollution.aspx
- Qiang Sun, Jiangjie Dong, Xuqiang Guo, Aixian Liu, Jingwen Zhang, Recovery of Hydrogen from Coke-Oven Gas by Forming Hydrate. Ind. Eng. Chem. Res. 2012, 51, 6205−6211.
- Thibaut Lepage, et. al. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment, Biomass and Bioenergy 144, 2021.
- Sakthivel, Shireesh S Swami, Atul Choudhari, Green Hydrogen: A Perspective. 35th Indian Engineering Congress on Engineering for Self-Reliance and Sustainable Goals, Technical Volume, 739-745, Dec 18th 20th, 2020 India.





