Jet Pathway: Production of Low-Carbon Jet Fuel from Alcohol

Introduction
Ethanol is an alcohol which can be produced either by petrochemical method though the hydration of ethylene or through biological processes like the fermentation of sugarcane, molasses, starch, wheat, corn etc. The biological process is considered to be world’s first sustainable biofuels, accounts for approximately 95 % of the ethanol production. Bio-ethanol is a leading renewable fuel used in the transportation sector due to it’s clean, affordable and low-carbon property. Ethanol alone is considered to be a low-cost alternative fuel for the environment than gasoline. This is because the bio-ethanol supplemented vehicles produce either lower carbon dioxide emissions or approximately the same or lower levels of hydrocarbon and oxides of nitrogen emissions. Hence, ethanol can be blended with gasoline and used in the automobile industry as a fuel.
Similar to the automobile industry, the aviation industry is also seeking technically viable and economical approaches to endowing sustainable alternatives to petroleum-based jet fuel. The major reasons for looking alternative jet fuel are cost in stability and climate change. There are two types of jet fuel such as Jet A or Jet A-1, which is most commonly used in commercial aviation industry. Other type is Jet B (i.e. naphtha-kerosene) which is commonly used in military aircrafts aviation flight. Mostly, Jet-A fuel is used (~22 billion-gallon per year) in the US, which has a freezing point of – 40 oC and Jet A-1 fuel is used (80 billion gallons per year) in the rest of the world, which has a lower freezing point of – 47 oC. Ethanol can also be converted into aviation turbine fuel (Jet A-1 fuel).
The alcohols cannot be directly used as aviation turbine fuels (called as jet fuel) due to its lower energy content (i.e. energy density of ethanol is 25 MJ/kg) and other properties such as flash point, freezing point, auto ignition temperature, when compared to the jet fuels namely Jet A and Jet A -1 (i.e. 43 – 48 MJ/kg).
There are many routes available for the production of jet fuel from renewable sources, such as
a) Hydroprocessed Esters & Fatty Acids (HEFA): The vegetable oils (triglyceride) and fatty acids are the most commonly used as feedstock.
b) Gasification and Fischer-Tropsch synthesis (FT): The syngas, which are derived from biomass, can be used as feedstock for the production of aviation turbine fuel.
c) Sugar to Jet fuel: It can be processed via two pathways such as (1) Catalytic upgrading of sugars and (2) Fermentation of sugars to produce liquid hydrocarbons products.
d) Alcohol to Jet fuel: Commonly, alcohol (e.g. ethanol / butanol) is used as feedstock.
This technical report discusses the Alcohol to Jet Fuel route and focuses on process description, opportunities, and challenges of jet fuel production from ethanol.
Ethanol to Jet fuel
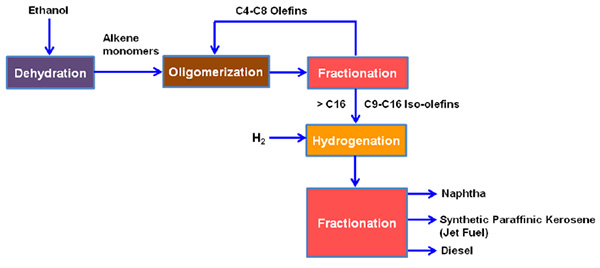
Typically, alcohol to jet fuel conversion process includes the following process steps
- Dehydration
- Oligomerization
- Hydrogenation
The dehydration, oligomerization and hydrogenation process steps are not new for chemical industries and this route is designed to rely on established and well-proven technology as shown in the Figure 1.
Figure 1 Schematic diagram of ethanol to Jet fuel
Dehydration
In dehydration stage, water molecule is removed from ethanol molecule through an acid catalytic reaction to produce ethylene. The basic reaction equation is as follows,
C2H5OH + catalyst → C2H4 + H2O
The thermal decomposition of ethanol to produce ethylene and water, takes place at a temperature range of 400°C to 450°C and at a pressure of 11 bar (approximately) in presence of alumina or transition metal oxides or H-ZSM5 zeolite catalyst. Higher temperature is required in order to shift the equilibrium toward product formation. There are a few side reactions that produce by-products like diethyl ether, acetaldehyde, propane and butane. These by-products could be removed through the separation unit to obtain high purity of ethylene product (99.96%). The by-product formation could be minimised by maintaining an optimal reaction temperature (400°C to 450°C).
Oligomerization
Oligomerisation process is conversion of ethylene (short-chain molecules) into linear α-olefins (long-chain molecules) via a catalytic reaction. Recently, there are many new catalysts are developed, such as Ziegler Natta, chromium diphosphine and zeolites etc. which can be used in the oligomerization process. Typically, the reaction takes place in the presence of sulfonic acid resins, solid phosphoric acid or acidic zeolites at a temperature range of ~ 100 to 300°C and high pressure. . The operating condition depends on the type of catalyst used and feedstock. The oligomerization of ethylene is given as follows,
n [C2H4] → C2nH4n
There are two commercially available processes of ethylene oligomerization. One of the processes is Gulf process or Ethyl-process, which is based on aluminium catalysts and the second one is Shell Higher Olefin Process.
1) Typically, Gulf-process produces linear α-olefins at temperatures above 200 °C and at a pressure of up to 250 bar in presence of tri-ethyl-aluminum (TEA) catalyst. This process which produce by-products in the range of 1.4 to 1.5 % of alkanes and branched α-olefins.
2) Ethyl-process is similar to the Gulf-process. The product stream is fractionated from C4, C6 to C10 and C12 to C16. The C4-fraction is further subjected to trans-alkylation, which increase the yield in higher alkenes.
3) SHOP-process involves liquid ethene as feedstock. The reaction occurs a temperature range of 80 °C to 120 °C at a pressure between 70 to 140 bar in presence of a nickel catalyst with phosphine ligands.
The selection of appropriate technology depends on several economic and site specific factors.
Hydrogenation
The α- olefins (C9-C16 and >C16) are subjected to hydrogenation process by addition of hydrogen in presence of nickel or palladium or platinum on activated carbon catalyst or zeolite support, which operates at temperature range of 300-400°C.
Challenges for ethanol to Jet fuel production
Following are the challenges for commercial scale operation
- Selection of technology to suit project objective
- Techno commercial feasibility
- Impact of feedstock variation & availability
- Estimation of yields at larger scales
- Process flexibility
- Understanding stability of catalysts, catalyst lifetimes, on-stream factors, and regeneration protocols and other critical process components
- Optimizing capital costs, which are especially challenging when new technologies are being developed
- Selection and optimization of separation technologies required to meet fuel specifications
Opportunities
Generally, the bio-ethanol is mainly produced in three ways such as sugar, starch, cellulose & hemicelluloses etc, which have high starch contents and non-molasses sources such as agricultural residues also. The feedstock of ethanol changes from country to country due to their soil and climate condition. In India, sugarcane molasses and straw of rice is the most common feedstock for bio-ethanol production. Recently, India has focusing on the production ethanol from bamboo feedstock also, which is available in north-east region of
India accounting 66 percent of the country’s bamboo resources. The feedstock of bamboo can convert into cellulosic ethanol, acetic acid, furfural and bio-coal, which would play a role in India’s energy security and promote green fuel use. Currently, India has producing ~1400 million litres of ethanol and it would be triple its ethanol production over the next four years till 2022. This could be encouraged the jet fuel production also. India bio-ethanol producer can think this direction.
Commercialization Readiness
Recently, ASTM International revised their ASTM D7566 Annex A5. The ethanol is added as feedstock for producing synthetic paraffinic kerosene (jet fuel). This revision clears the way for increased adoption of sustainable aviation fuels, because ethanol feedstocks can be made from so many different low-cost sources. There are several demonstration plants installed worldwide and the produced jet fuel has been tested with major airlines. Many companies have reported development of processes to produce Jet fuel from ethanol, but its economics depend mainly upon the source from which ethanol is produced. Currently, ethanol has relative advantage in terms of market price over other alcohols like butanol.
The feedstock costs of alcohol, which contributes +30% of jet fuel price, is considered to be the greatest impediment to commercialization of jet fuel. The ‘Alcohol to Jet Fuel’ process costs consists two processes, such as biomass to ethanol process and conversion of ethanol to jet fuel via dehydration, oligomerization & hydrotreating. The capital costs of producing bio-ethanol feedstock are function of amount of feedstock and their price. The capital costs of ‘Alcohol to Jet Fuel’ are considered as a linear function cost of size of facility. Currently, the world’s first large scale facility of significant capacity, will be coming up in the UK. The commercial facility would convert low carbon ethanol to jet fuel.
Closing remarks
The ‘Alcohol to Jet Fuel’ process is one of the promising emerging technology to make jet fuel. This article presented brief overview of the process description, operating condition of the process, challenges, opportunities and environmental benefits etc. This process mainly aims to reduce petroleum consumption and also to reduce greenhouse gas emissions as compared to the petroleum based jet fuel. The feedstock of alcohol production cost is considered to be the greatest barrier to commercialization of this process. However, India has great potential to produce ethanol from bio resources, which shows good opportunities of ethanol producer to convert this feedstock into jet fuel.